Biocidal Product Regulations - Overview
Biocidal Products are Disinfectants/Antimicrobial products that have gained new meaning and purpose with the spur of the COVID-19 pandemic. These products are called/identified and regulated differently across countries and regions making it very challenging for the manufacturers to market the products.
Different terms and Regulatory classifications applicable for these products include chemical disinfectants/HUHS/biocide/pesticide/OTC/quasi drug/antimicrobial pesticide and even Consumer Products, Cosmetics, Medical Devices, and Medicinal Products. In most markets, product classification and regulations are driven by the chemical composition and the claims made on the product label or other advertisement and promotional material.
Biocidal product regulations in some countries have a clear framework with a defined registration process and timeline. While many countries do not have well-defined structures and processes, resulting in uncertainties, duplication of data, and delays in market access. Further, chemical regulations are dynamic and are evolving, considering new scientific data and sustainability pressure.
The other key factors for market access and compliance are provided below:
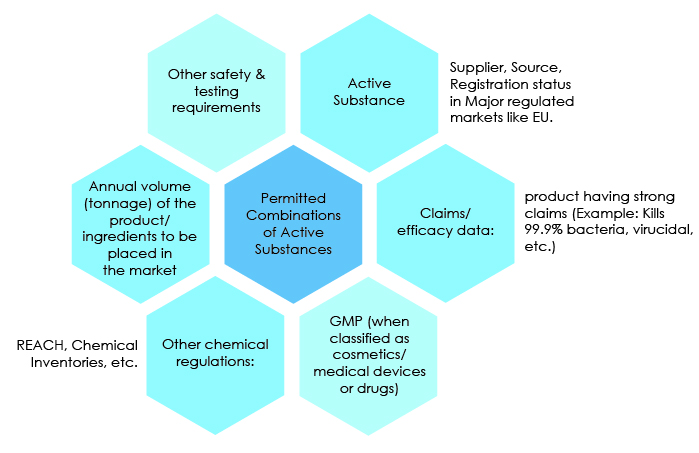
Table 1: Regulatory Summaries for Major Markets
|
Country |
Classification |
Authority
|
Registration/Notification |
Active Substance Supplier Requirements |
Requirements |
Standards |
Timelines |
|
Europe |
Biocides |
ECHA & associated individual member state (EU 27)/EU BPR 528/2012 transitional measure (article 89) & harmonized authorization |
Yes |
Active substance suppliers should be approved suppliers mentioned in the Article 95 list. |
Efficacy/ Chemistry/CLP requirements |
CEN/OECD & other international standards |
Transitional measure: one (01) month to one (01) year (depends on EU MS) Authorization: three (03) months to 1.5 years (depending on the type of authorization) |
|
US |
Antimicrobial Pesticide (surface disinfectant used on inanimate objects) |
US EPA under Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA) |
Yes |
The US EPA-approved active ingredient & supplier |
Efficacy/ Chemistry/ Toxicology & GHS requirements |
EPA standards and other international standards fulfilling the requirements |
Six (06) months to twelve (12) months |
|
Canada |
Disinfectant Drugs/Pesticide (HS code – 380894) |
Natural and Non-prescription Health Products Directorate (NNHPD) & PMRA |
Yes |
None |
Efficacy/ Safety & Quality |
ASTM & other international standards fulfilling the requirements |
One (01) to two (02) years (depending on the submission type) |
|
South Africa |
Chemical Disinfectant |
Surface Disinfectant (environment surfaces/ inanimate objects) to be registered by the National Regulator for Compulsory Specifications (NRCS) following SANAS standards |
Yes |
None |
Efficacy/ Chemistry/ Safety and GHS requirements |
SANAS standards and other international standards fulfilling the requirements |
Approximately six (06) months |
|
Singapore |
General Consumer Product |
Health Science Agency |
No |
None |
Consumer goods safety requirement compliance |
International and SS standards |
Not Applicable |
|
UK |
Biocide |
GB Biocidal Product Regulation (BPR) |
Yes |
GB article 95 listed suppliers only |
Efficacy/ Chemistry/Safety and CLP requirements |
CEN standards and other international standards fulfilling the requirements |
Three (03) months to 1.5 years (depending on the type of authorization) |
|
Philippines |
Category 3 HUHS (household urban pesticides) |
FDA circular 2020-025 listing via FDA e-portal. |
Yes |
None |
Efficacy/ Chemistry/ Safety and GHS requirements |
CEN and other international standards fulfilling the requirements |
Three (03) months (approx.) |
|
Malaysia |
Surface Disinfectant (used on non-porous surfaces) (HS code – 380894) |
National Pharmaceutical Regulatory Agency (NPRA) registration |
No (Import Licence may be required) |
None |
Efficacy/ Chemistry/ Toxicology |
ECHA/ EPA accepted standards other international standards fulfilling the requirements |
Four (04) to six (06) months (approx.) |
|
UAE |
Biocide |
Dubai Municipality and Health Safety Department |
Yes |
None |
Chemistry/Safety/ Efficacy |
EU and other international standards |
Up to one (01) week from the application completion date |
To place a biocidal product in any market or set of markets, companies are first required to build robust strategies to comply with market-specific regulations and exploit overlapping testing and certification requirements to minimize cost and time-to-market.
Freyr has extensive expertise in providing Biocidal product classification and Regulatory solutions across the global markets.
Biocidal Product Regulations - FREYR EXPERTISE
- Categorization of Consumer Products.
- Developing Product Notification/Registration Strategies.
- Regulatory Applicability Review and Interpretation.
- Preparation of Notification Dossiers.
- Application Preparation & Submission for Relevant Introductions.
- Sustainability & Packaging Solutions.
- Regulatory Intelligence Support.
Biocidal Product Regulations - FREYR ADVANTAGES
- End-to-end chemical Regulatory services.
- Expert advice on Regulatory strategy.
- Easily navigate the complexities of Regulatory authorities.
- Support for region-specific Regulatory complexities.
- A structured and cost-effective approach to ensure compliance.
- Robust partner network in Australia.
- Focused compliance solution.
- Quick turnarounds and faster time-to-market.