Chemical Registration in Philippines - Overview
Here are a few key facts for you!
- Household Urban Hazardous Substances (HUHS) products are divided into five (05) categories, and Surface disinfectants/Detergents-cleaners fall under Category III.
- The products in Category III can be classified as ready-to-use products and professional-use (highly-concentrated) products.
- Household Urban Hazardous Substances (HUHS) products cover a large variety of products range (mentioned in Annex A of the FDA circular 2020-025) except pharma, cosmetics, and medical devices.
General Requirements for Household Urban Hazardous Substances (HUHS) Products (Surface Disinfectant/Detergent-Cleaners)
- Shall be classified into the relevant category.
- Shall not contain CMR/banned ingredient.
- Shall not bear unsubstantiated claims or misleading information.
- HUHS manufacturers should follow good manufacturing practices.
- Product Information File (PIF) shall be maintained and kept ready post-registration for inspection purposes.
“In case you are a foreign company who is exporting HUHS product (Surface disinfectant/ detergent-cleaners) to the Philippines, make sure your distributor-importer hold a valid License to operate (LTO) in order to apply for company product registration and to clear customs”
License-To-Operate (LTO) for HUHS Establishments – The Philippines
License-To-Operate (LTO) for HUHS establishment is a crucial document to hold in the Philippines by local establishments like distributor-importer before getting engaged in any activity related to HUHS products like marketing/distribution/importation/export/sale and offer for sale/promotion, etc. & to apply for HUHS chemical registration in Philippines through FDA. Further, LTO & CPR are equally important and required by the Bureau of Customs (BOC) to clear customs.
Applicants can apply for LTO to FDA through the FDA e-portal system V.2, which takes 20 – 30 days of time to process the application, where major data requirements would be:
- Declaration and Oath of Undertaking and Accomplished Application Form.
- Proof of Business Name Registration.
- Proof of Income.
- Fee Payment.
The initial validity of the LTO is for three (03) years, and after renewal, the validity is extended for five (05) years, unless revoked.
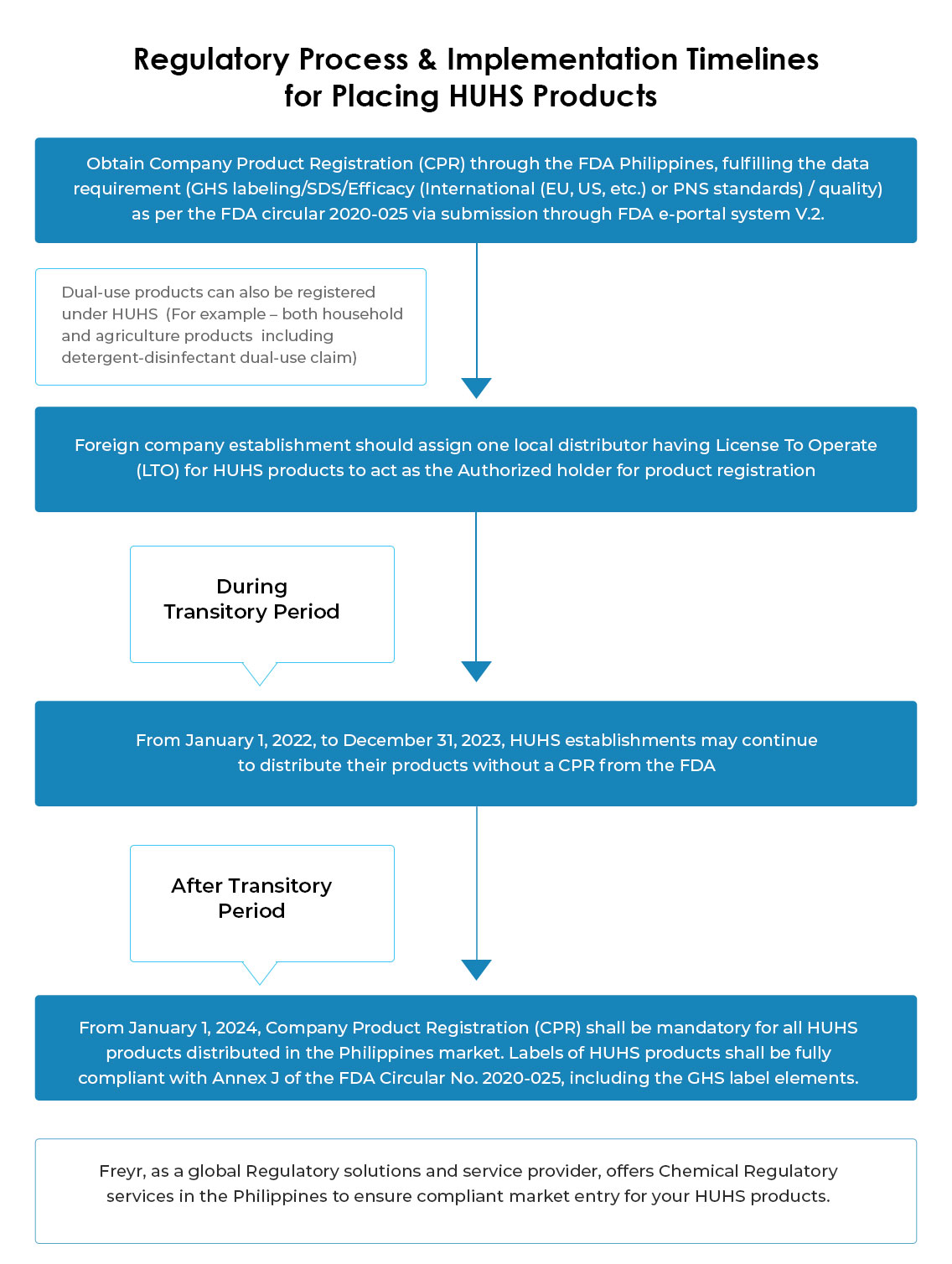
Under the transitory period (Jan 01, 2022, to Dec 31, 2023), extension is not applied to the License To Operate (LTO), hence, from Jan 01, 2022, LTO as HUHS establishments shall be mandatory for all establishments engaged or intending to engage in HUHS-related activities.
Chemical Registration in Philippines - FREYR EXPERTISE
- Company Product Registration (CPR) services:
- Product classification.
- Building data requirements matrix.
- Data collection and data gaps validation.
- Preparation of declaration statements & application forms.
- Application submission via FDA e-portal.
- Post-submission support - Quick responses to authority-raised queries before the assigned deadlines.
- Follow up till approval and approval validation.
- License To Operate (LTO) Application Submission.
- LTO - Legal Representative & Authorized Holder Services.
- Regulatory Intelligence (RI) Support.
Chemical Registration in Philippines - FREYR ADVANTAGES
- End-to-end chemical Regulatory services.
- Expert advice on the Regulatory strategy.
- Easily navigate the complexities of Regulatory authorities.
- Support for region-specific Regulatory complexities.
- A structured and cost-effective approach to ensure regulatory compliance.
- Robust partner network in the Philippines.
- Focused chemical Regulatory compliance solution.
- Quick turnarounds and faster time-to-market.